Hydrogen cars are powered by fuel cells that are dependent on platinum. Catalytic research shows that the amount of platinum can be reduced. Platinum may even be replaceable.
Emma Westsson (right) and Ger Koper in the lab. (Photo: Jos Wassink)
In fuel cells, hydrogen (H2) and oxygen from the air (O2) combine to form water and electrical power. It sounds easy, but advanced membranes and platinum catalysts are required to get the process going. And although the amount of platinum needed has been reduced to 10 grams per car, the costs of platinum still make up 30% of the fuel cell production costs.
According to Dr Emma Westsson, who did her PhD research on fuel cell catalysts, fuel cell manufacturers call the need for platinum ‘an urgent concern’. Not only because of the platinum price (currently about USD 30/gram), but also because of the geopolitical dependence. The noble metal platinum is only found in two places in the world: South Africa and Russia.
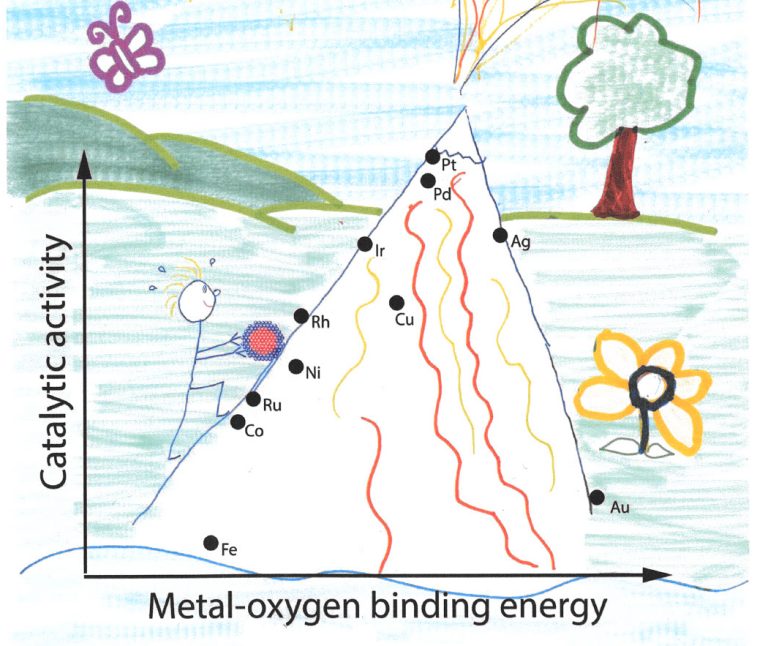

A good catalyst is like a Post-it memo: it is sticky enough, but not too much. The Danish researcher Jens Nørskov developed a graph showing the attractiveness of catalysts in the form of a volcano. As the stickiness to oxygen decreases (from left to right), catalytic activity goes up. But after platinum (Pt at the peak), the stickiness becomes too weak and the catalytic activity goes down. In other words: the product sticks to the catalyst too loosely.
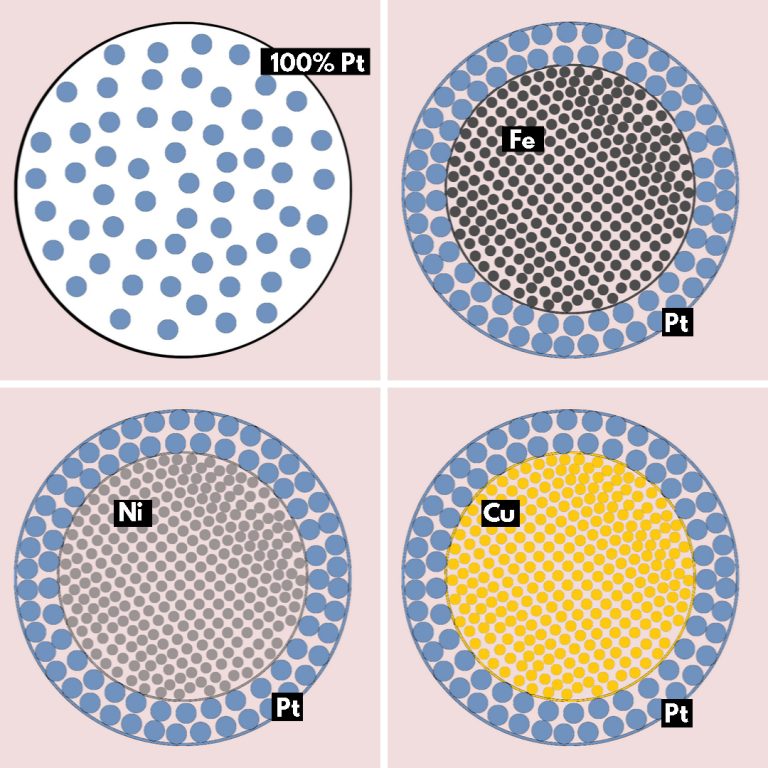

To reduce the need for platinum, Westsson developed catalyst particles like Smarties. The outside is still platinum, but inside is another metal. The particles are 2-3 nanometres wide, just a couple of hundred atoms in size. Her PhD supervisor Dr Ger Koper (Faculty of Applied Sciences) estimates the coated metal particles reduce the amount of platinum by 50%. According to Westsson, the catalytic activity for iron, nickel and copper cores is the same or better than pure platinum nanospheres.
Fitting the platinum layer introduces strain in the lattice. First, because Pt atoms (atomic number 78) are bigger than the atoms at the core (Fe (26), Ni (28) or Cu (29)). They get squeezed as they attach to the smaller lattice. Second, the curvature of the nanosphere introduces extra strain as well.
Strain is fine for catalysts, found Westsson. Catalytic activity increases with strain in coated metal particles with nickel cores, she showed.
“She has developed tuneable catalysts,” says Koper. “The catalytic activity can be tuned by using another metal core, or by changing the lattice strain.” That was fundamental science. “We leave it up to companies to find the most favourable combination.”
Westsson thinks that careful tuning of other less rare metals will allow them to take the place of platinum at the top of the Nørskov curve. She writes: ‘Other metals than Pt could be studied and possibly take Platinum’s place as the most active oxygen reduction catalyst’.
Five years ago, the same research group led by Professor Stephen Picken developed carbon nano networks for use in fuel cells.

Emma Westsson, Low Noble Metal Content Catalysts for Hydrogen Fuel Technology, PhD supervisors Professor Stephen Picken and Dr Ger Koper (Faculty of Applied Sciences), 20 September 2019.
Do you have a question or comment about this article?
j.w.wassink@tudelft.nl


Comments are closed.