Easily produced carbon nano networks may make fuel cells less expensive, says PhD student Emanuela Negro (faculty of Applied Sciences).
Fuel cells that convert hydrogen (or other gases) and oxygen directly into electricity are the paragon of efficient clean technology. Their efficiency mounts to 60 percent (90 if the heat is used as well) and the only ‘waste’ is pure water.
Yet, the electrodes for the electrochemical reactions are still in development since they need to combine a number of properties that are hard to combine. Next to the conduction properties of protons and electrons, they need a large surface where the reactions take place and a very porous structure for the gases to pass through. Plus the electrodes need to be resistant to corrosion – the main cause of performance degradation in low-temperature fuel cells.
A new material called carbon nano networks (CNN’s) seems to offer promising properties. “We compared it to the performance of carbon nanotubes containing platinum nanoparticles and we compared how well it works with non-platinum catalysts”, says Emuela Negro – a chemical engineer from Turin with a degree from KTH in Stockholm as well.
“We found carbon nanonetworks are resistant to corrosion, they provide a high surface area and a good binding of the catalysts. In other words: it performs well as an electrode material, but not exceptionally well.”
The real advantage of carbon nano networks may be their easy manufacture. Dr. Ger Koper and Dr. Krishna Kowlgi in Professor Jan van Esch’s group for ‘advanced soft matter’ (at Applied Sciences) developed a technology in which metal nanoparticles in a soap-like coating are finely dispersed in an emulsion of water and oil. When placed in an oven (at about 700 degrees celsius in a inert atmosphere) carbon rod-like nanostructures start forming at the nanoparticles. When a carbon-rich gas (like ethene C2H4) is fed, the rods start deforming and growing cross connections. This patented synthesis precursor distinguishes nano networks from other graphitic materials (TU start-up CarbonX explores carbon nano network in polymers for construction, energy, aerospace and sports).
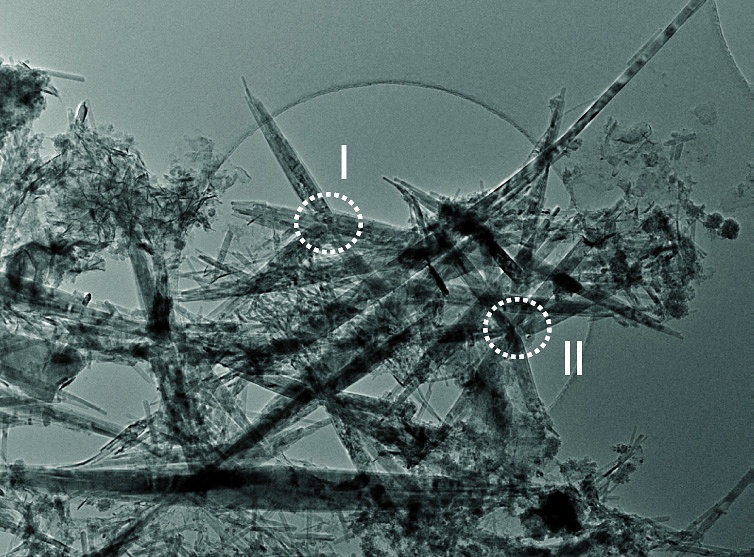

Negro found that nano networks can be directly grown on carbon paper – a very common and cheap base material for electrodes in fuel cells . Under the electron microscope it looks like the smooth long carbon fibers from the carbon paper have suddenly grown spines. On this high surface area support, catalysts (such as platinum) where gas conversion takes place can be deposited .
“Manufacture may easily be scaled up”, thinks Negro. “You just need to roll your catalyst on top of the carbon paper, and put it in the oven to grow your networks.” Even though carbon nano networks do not improve the performance of fuel cells, they may make them less expensive and more durable.
–> Emanuela Negro, Conductive graphitic networks: from atoms to fuel cells, PhD thesis supervisors Dr. Ger Koper, Prof. Jan van Esch and Prof. Stephen Picken (Applied Sciences), Defense 14 November 2014. Research founded by the Dutch programme ‘A green Deal in Materials’ (ADEM).



Comments are closed.