The biotech tool CRISPR-Cas has caused a revolution in genetic editing. In a recent issue of Science, TU researcher Stan Brouns argues that the biotech revolution has only just begun.
The CRISPR-Cas system has been in use for genetic editing since 2013. It allows scientists to rewrite DNA sequences in living cells with unprecedented precision. Thousands of researchers worldwide use the technique to delete or replace genes in living organisms. The medical and biotechnological potential of the CRISPR technology is hard to overestimate.
TU researcher Stan Brouns (Department of Bionano science at the Faculty of Applied Sciences) is one of the authors of the review article CRISPR-Cas: Adapting to change in Science (April 7, 2017). Brouns wrote the article together with colleagues from the University of Otago (New Zealand) and Wageningen University.
Accomplishments
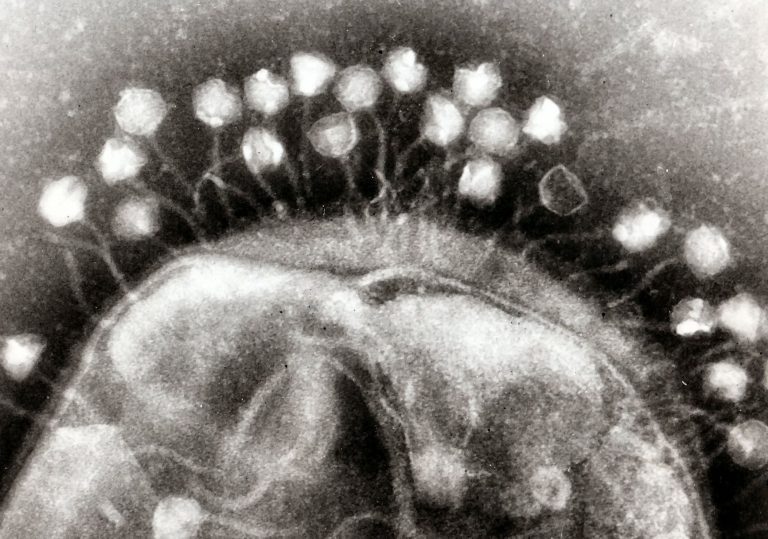
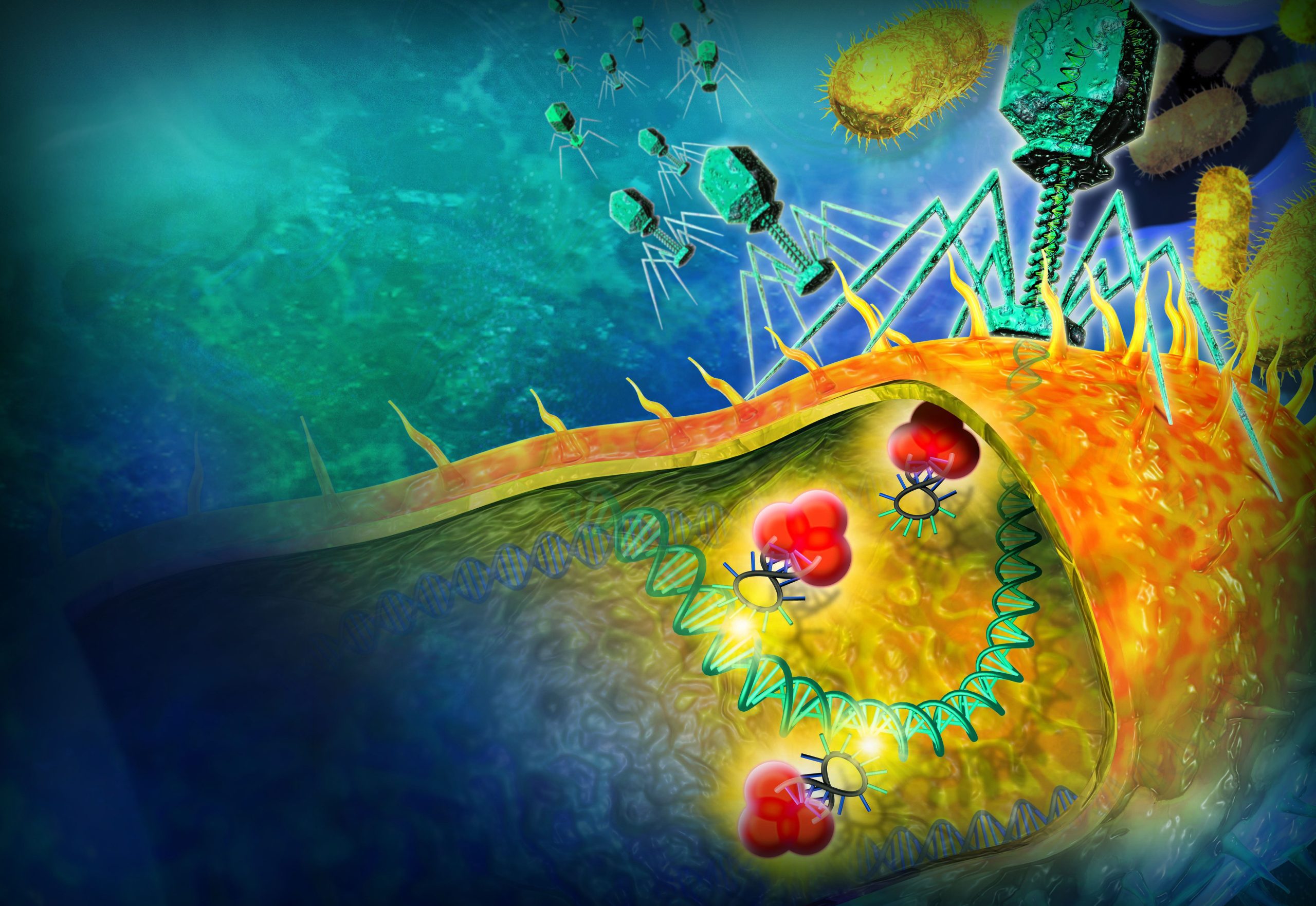
The article starts out not with the latest accomplishments in gene editing, but with the eternal molecular arms race between bacteria and viruses. In this microbial world, CRISPR-Cas evolved as a bacterial defence mechanism against viruses. The acronym stands for Clustered Regularly Interspaced Short Palindromic Repeats and associated proteins.
“In the article, we highlight the recent advances in the understanding of the molecular mechanisms of how these bacterial defence systems form memories against their virus invaders”, said Brouns.
Toolbox
One of the reasons for the article’s fundamental approach is that CRISPR involves much more than the mechanism that is currently in use. Brouns: “CRISPR-Cas is not one thing, but rather a whole toolbox.” So much more reason for Brouns and colleagues to want to fully understand the fundamental biology behind it.
CRISPR-Cas has evolved in bacteria as an adaptive immune system against viruses (bacteriophages). A bacterium that survives a viral attack takes a sample of the viral DNA and stores in in its own genome between repeated sequences. This genomic memory allows the bacterium (and its descendants) to recognise the virus later on, and cut its DNA into pieces.
Workhorse
This bacterial defence mechanism has been hijacked by scientists to edit DNA sequences. In particular, the Cas-9 protein has proved to be useful to insert a new piece of DNA into a chromosome. Cas1-Cas2 are known as the workhorse for the formation of bad viral memories between the repeat sequences.
According to Brouns and colleagues, this is only the beginning. “As we begin to comprehend CRIPSR adaptation in more detail,” they write, “the opportunities to repurpose other parts of these remarkable (…) immune systems are increasingly becoming a reality.”
DOI: 10.1126/science.aal5056
Related article in Delta: Revolutie in de DNA-wereld


Comments are closed.