As a rapid succession of electric sparks generates nanosized metal particles, it opens the way to produce new energy materials in bulk, discovered Tobias Pfeiffer in his PhD research.
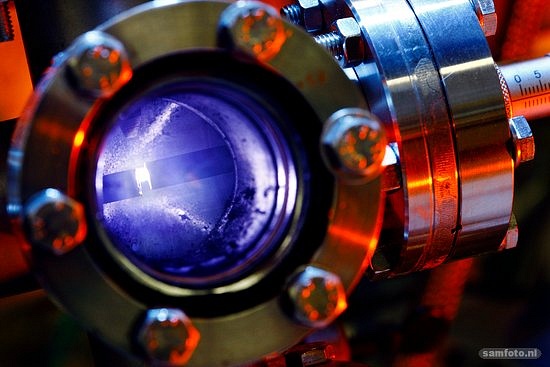
A soaring white light bursts from the looking glass as 25 thousand sparks per second flash between the electrodes. They’re safely tucked away in a stainless steel tube, flushed by a gas that carries away the vapour cloud from the electric plasma with core temperatures of 20 to 60 thousand degrees. The high temperature knocks nano-sized particles (single atoms to several tens of nanometres) from the electrodes in a process called ‘ablation’.
In the aerosol phase, these nanoparticles can be manipulated and modified in a myriad of ways. They can be left to agglomerate in fluffy structures, used in chemical reactions, selected on basis of their size, or deposited onto a substrate to make functional materials with new macroscopic properties.


“This is a whole new way of doing chemistry”, says Pfeiffer (ChemE, faculty of Applied Sciences) “which enables new combinations of most every metal in the periodic table. It does so without using solvents or producing waste. And it produces nanomaterials not by milligrams, but by grams or even kilograms per day.”
No wonder then that Pfeiffer has patented parts of the sparking process and will start producing nanoparticles in a start-up company called VSParticles (very small particles) with two partners after his PhD thesis defence.
Among the nanoparticles that Pfeiffer produced are silver nanospheres of typically 100 nanometres diameter. The sparked particles are smaller, but they’re left to cling together (‘to agglomerate’) after which they’re sintered into spheres at 800 degrees celsius. When deposited in the top layer of photovoltaic (PV) cells, the silver particles trap the light within the plane of the cell by scattering it and thus increase the maximum current of the cell. With Rudi Santbergen of EEMCS faculty, Pfeiffer demonstrated an increase of a PV short-circuit current by 4 to 15 per cent, depending on the spheres’ size.
Another ‘functional’ energy material profiting from spark synthesis is magnesium hydride (MgH2). The material is in vogue because of its capacity to reversibly bind and release hydrogen, which may be an interesting option for hydrogen storage without having to resort to extremely low temperatures or high pressures.
“The trouble is that the hydride seals the magnesium, preventing any more hydrogen to bind. It’s only the outer 20 nanometres that are involved in hydrogen-binding”, explains Pfeiffer. Therefore it would make sense to spark-produce magnesium hydride nanoparticles. In practice, the product carried by hydrogen and argon gas passing in between two sparking magnesium electrodes was no pure magnesium hydride, but also contained magnesium and magnesiumoxide . At 4,3 per cent maximum, the content is 43 grammes H2 per kilogram magnesium hydroxide.


Another critical point is the energy-intensity of the sparking chemistry: producing a gram of nanoparticles typically costs several million joule/gram or up to 10 kilowatt-hours.
Pfeiffer is not overly worried by the energy costs. He points out that the growing share of renewable sources in the energy mix often results in overproduction. “The excess power can be converted into the production of nanoparticles, which in turn enable the production of new energy materials such as better PV cells or hydrogen storage materials”, he joyfully explains.
–> Tobias Vincent Pfeiffer, Towards the industrial application of spark ablation for nanostructured functional materials, 13 October 2014, PhD supervisor Prof. Andreas Schmidt-Ott (AS).


Comments are closed.