A research consortium that includes Prof. Cees Dekker’s group has shown the active role of the condensin protein in coiling long DNA strings. Its result, published in Science, ends a long-standing debate about the working of the protein.
The condensin protein has a vital function in coiling a cell’s DNA. It is baffling that each cell in your body, with a typical diameter of 0.01 millimetre, contains two metres of DNA string, jumbled together like a plate of spaghetti. Each time a cell divides, the DNA is coiled up or condensed in 23 sets of double chromosomes. Scientists know that condensin plays a vital role in the coiling of DNA, but are divided over the mechanism.
One group says that condensin hooks up on two spots somewhere along the DNA and binds them together like a tie-wrap. The other group argues that condensin has a motor function. Once the ring-shaped nano-engine is attached on the DNA-string, it actively transports a large piece of string through the ring and thus forms a loop.
Nanobiologist Professor Cees Dekker, one of the authors of the Science article and research leader of the Kavli Institute for Bionanoscience, says: “The trouble with this so-called loop extrusion model is that the motor function has never been observed. Also, people have calculated that it would take much more energy to pull the string through the ring step-by-step than has been observed as energy use.”

In a clever and decisive experiment, PhD student Jorine Eeftens, has shown that condensin proteins, marked with a fluorescent quantum dot, quickly move along fixed DNA strings as long as there is fuel, in the form of ATP molecules, present. She registered velocities of about 60 base pairs per second and distances travelled more than 10,000 base pairs.
When a fluorescent piece of DNA was attached to the condensin protein instead of to a quantum dot, the displacement remained unchanged. This observation means that condensin can actively transport one piece of DNA relative to another, something that fits well into the active loop-forming theory.
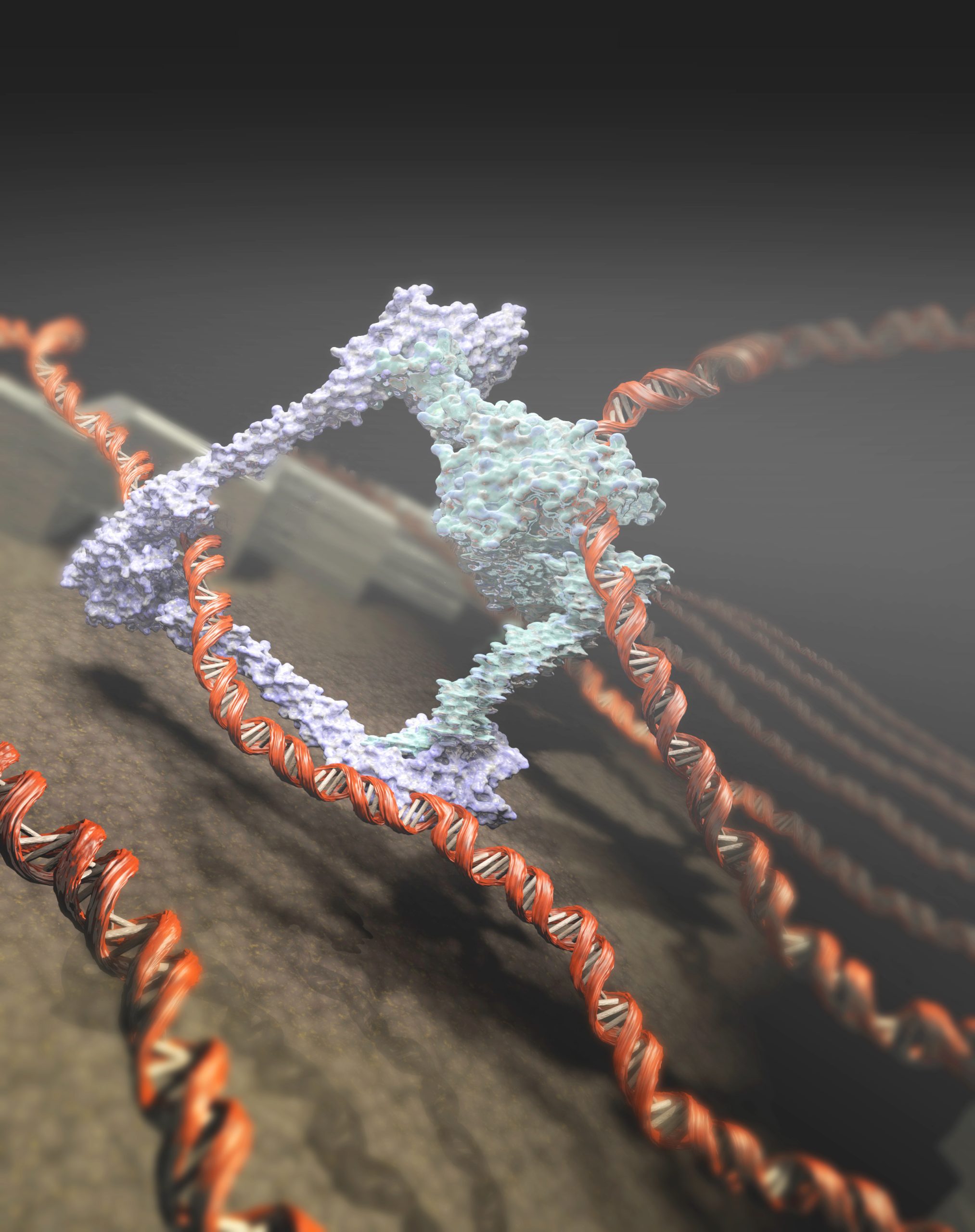 Condensin protein. (Image: Tremani and Cees Dekker Lab TU Delft)
Condensin protein. (Image: Tremani and Cees Dekker Lab TU Delft)Dekker comments: “The precise mechanism of how DNA loops are formed remains unknown. But this discovery definitely supports the theory of the active loop-forming. Besides, we have shown that the energy use by the condensin complex can be much lower than previously thought.”
For this project, the Kavli Institute for Nanoscience Delft worked with the European Molecular Biology Laboratory (EMBL) in Heidelberg and the Department of Biochemistry and Molecular Biophysics at Columbia University in New York. The research was funded by the NWO Zwaartekracht programme, ‘Frontiers of Nanoscience’.
• Tsuyoshi Terakawa, Shveta Bisht, Jorine M. Eeftens, Cees Dekker, Christian H. Haering, Eric. C. Greene, The condensin complex is a mechanochemical motor that translocates along DNA, Science, 7 September 2017.
doi: 10.1126/science.aan6516
Do you have a question or comment about this article?
j.w.wassink@tudelft.nl

Comments are closed.