The European Research Council has awarded ERC Starting Grants worth 1.5 million euros each to four TU Delft researchers. Dr Wilson Smith is one of the lucky four. He will expand his research on electrochemistry.
Smith’s research group at the Department of Chemical Engineering, focuses on understanding and optimising the conversion and storage of solar energy using materials and chemicals that are abundant on earth. Or, as Smith writes on his website, “we convert sunlight into electricity and chemical fuels”.
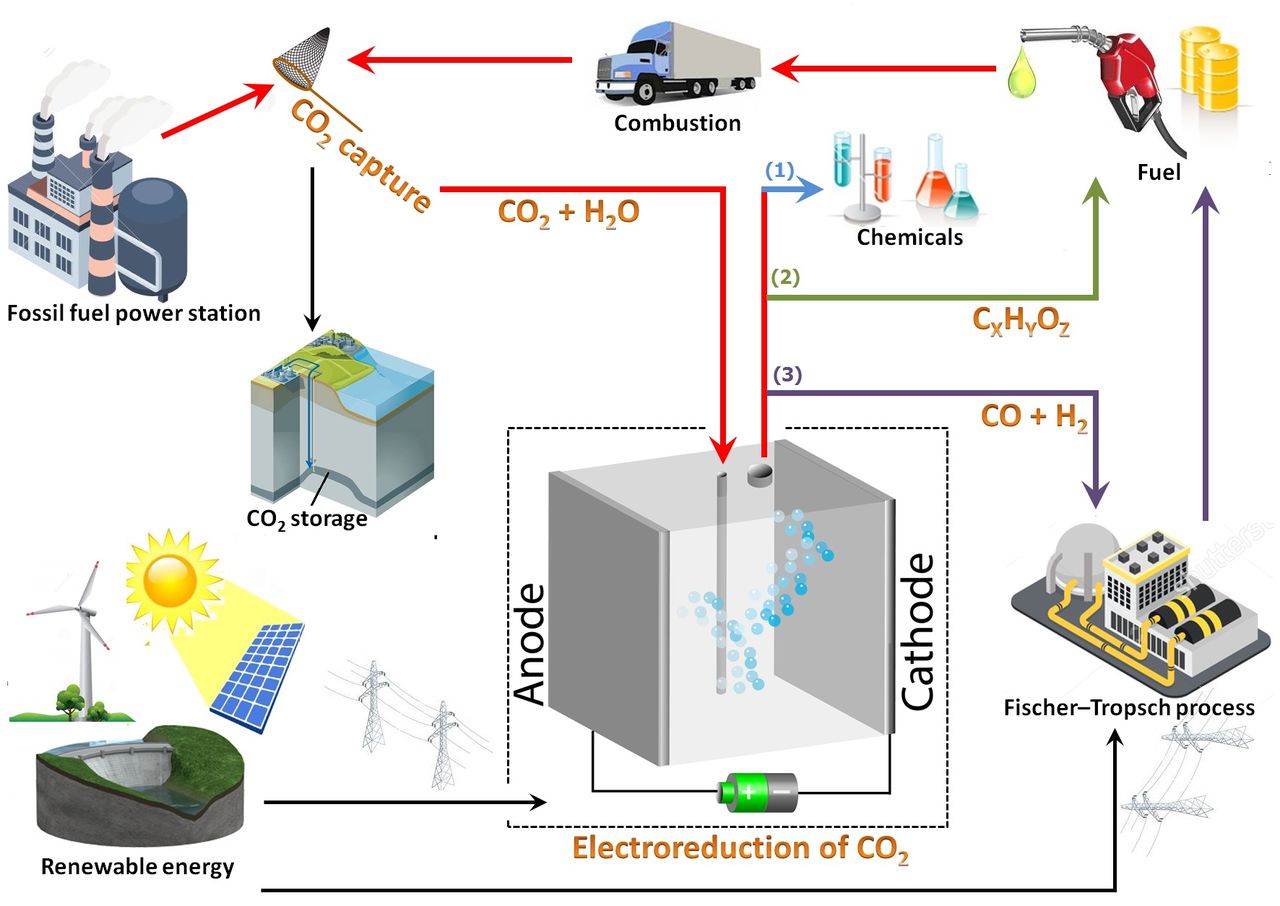
One area Smith is working on is to find a way to precisely control the process of electroreduction of CO2 to produce a wide range of useful products. This is called carbon capture and utilisation (CCU).
It is a shame to store CO2 in the ground in an effort to mitigate climate change, like the capture and sequestration movement is proposing. Carbon capture and utilisation could be a feasible alternative strategy for mitigating atmospheric CO2 concentration, or so Smith and his colleagues write in a press release. In this process, the captured CO2 is used as a resource and is converted into carbon monoxide, methane, ethylene, and even liquid products. Last week, the first PhD student from Smith’s group, Dr Ming Ma, defended his thesis. Ma has found a way to produce alcohol out of thin air.
The high energy density hydrocarbons can be utilised as fuels in the current energy infrastructure. In addition, the production of carbon monoxide (CO) has great potential since it can be used as feedstock in the Fischer–Tropsch process, a well-developed technology that has been widely used in industry to convert syngas (CO) and hydrogen (H2) into valuable chemicals such as methanol and synthetic fuels.
“The electrochemical reduction of water and CO2 can form at least twenty different products which depend on the catalyst surface, the potential applied, and the electrolyte used,” writes Smith. “In our research, we focus on nanostructuring the surface of metal catalysts such as copper and silver to change the selectivity of the CO2 reduction reaction, while also enhancing the rate of the reaction.”
But for now, CO2 reduction is not yet ready for large-scale deployment due to the poor activity and selectivity of the catalysts that are currently used.
In this project, Wilson Smith will use a variety of techniques and computational methods to gain fundamental insights into the reaction pathway of electrochemical CO2 reduction. He will receive an additional 500,000 euros to buy a scanning electrochemical microscope which will allow him to measure the electrochemical behaviour of materials.
The three other ERC Grants receivers are Liedewij Laan, Monique van der Veen and Manuel Mazo. Laan will be researching how adaptive mutations in yeast cells improve their fitness, to help us get a better insight into the evolution of organisms. Van der Veen’s project is aimed at designing ferroelectric materials. And Mazo is looking to reduce the cost of implementation and maintenance of so-called cyber-physical systems, which are digital systems that regulate complex physical processes, such as chemical reactors or power networks.
Do you have a question or comment about this article?
tomas.vandijk@tudelft.nl

Comments are closed.