Bacterial infections are becoming increasingly resistant to antibiotics. Bateriophages could offer an alternative. TU Delft Phage Library makes an inventory.
The Phage Library Team, Stan Brouns second from left. (Photo: Sam Rentmeester)
The TU Delft Phage Library (Fagenbank) will be opened during the Medical Delta Conference, the annual gathering on medical technology, on 16 April 2019. The Phage Library is an initiative of microbiologist Dr Stan Brouns (Faculty of Applied Sciences). He is working on developing bacteriophages as an alternative to antibiotics.
In Europe, 33,000 people die every year from infections of resistant bacteria. Experts believe that by 2050, worldwide 10 million people will die from bacterial infections every year. So, alternative ways to treat bacterial infections are urgently needed. But how effective are these bacteriophages? And what are the side effects?
What are bacteriophages
Bacteriophages are viruses that attack and kill bacteria. Put yourself in the micro-world for a moment. All around you hover bacteria no more than 1-3 micrometres across. Among them swim bazooka shaped structures that grope for prey. These are bacteriophages that have been bacteria’s natural companions for billions of years. They have evolved together. Generation after generation they have trumped each other’s defences.
So when a bacteriophage recognises a suitable host, the bazooka’s barrel will lock onto the bacterium’s membrane. This action triggers a mechanism that forcefully releases the genetic content of the phage’s head into the bacterium. The bazooka ejects its load. A phage may only be one 10th of the dimension of its host, but it is sublimely suited for its sole purpose in life: getting a bacterium to make as many copies of itself – the phage – as possible.
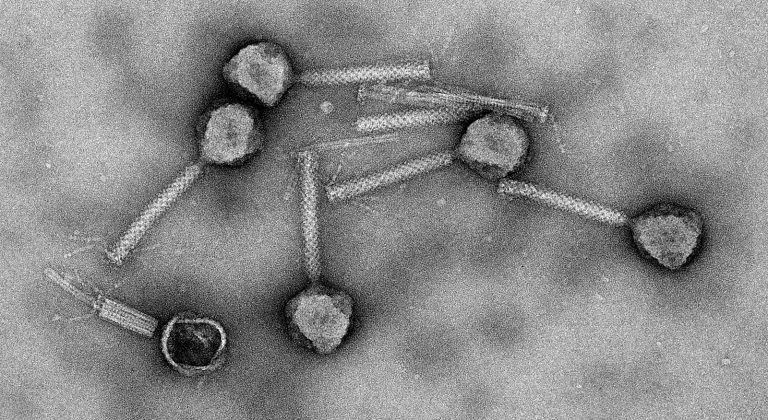 Electron microscope photo showing bacteriophages. (Photo: TU Delft)
Electron microscope photo showing bacteriophages. (Photo: TU Delft)“These are the ultimate nano machines,” says Brouns during the interview in the spectral coloured AS building. “These machines are dozens to hundreds of times more sophisticated than the current state of our nanotechnology.”
And yet, these sophisticated nano machines have very humble origins. They mainly come from bowels, sewers and waste water treatment plants. It’s funny to think that the frozen and carefully labelled collection at the clinical white Phage Library has such pungent origins.
Chicken typhoid
About 100 years ago, the English bacteriologist Frederick Twort and the French-Canadian Felix d’Hérelle discovered the anti-bacterial effect of certain substances. They observed the effect of an ‘invisible antagonistic microbe from the dysentery bacillus’. In early 1919, d’Hérelle isolated bacteriophages from a filtrate of chicken shit. And he successfully applied it to cure chicken typhoid. His success bolstered his confidence to such an extent that he decided to release bacteriophages on humans as well. In August 1919, he used phages to cure the first patient of dysentery, and many more cured patients followed.
Bacteriophage: that which eats bacteria
Although no one at that time knew what a bacteriophage was (literally: that which eats bacteria), d’Hérelle was not far off with his description of ‘a biological organism that preys on bacteria’. In fact, he would be excited to see the electron microscope photos of phages as specialised nano machines hunting bacteria.
D’Hérelle acquired fame all over Europe. He was awarded an Honorary Doctorate from Leiden University in 1924, and a Van Leeuwenhoek medal from the Royal Dutch Academy of Sciences (KNAW). Despite this display of honours, bacteriophages were largely forgotten in Western Europe after the Second World War and the introduction of antibiotics.
But on the other side of the Iron Curtain, in Poland and Georgia especially, bacteriophages were cultured for medical applications. The world famous Phage Therapy Centre in Tblisi, which d’Hérelle himself visited, has been bought by an American company.
Establish what works
The story of bacteriophages has meandered between hope and fear, promise and scepticism, but lately, curiosity seems to be taking over. “We want to overcome doctor’s suspicions,” says Brouns about his Phage Library. “We want to establish what works and what does not. And what the risks are.”
 Bateriophages are kept in a freezer. (Photo: TU Delft)
Bateriophages are kept in a freezer. (Photo: TU Delft)“We are working on a collection of phages against the 15 most resistant bacteria,” says Brouns. That means culturing phages in bacterial colonies and filtering the phages from the solution. The phages are kept in small vials in the freezer, each and every lid neatly marked with a unique code.
 Bacterial colony. (Photo: TU Delft)
Bacterial colony. (Photo: TU Delft)The effectiveness of phages against bacterial growth is recorded in a matrix of phages versus bacteria. Green means the phages win (and kill the bacteria), yellow indicate a reduction in growth, or white if there is no discernible effect. Thus, the team systematically builds up knowledge of which phage is effective against which bacteria.
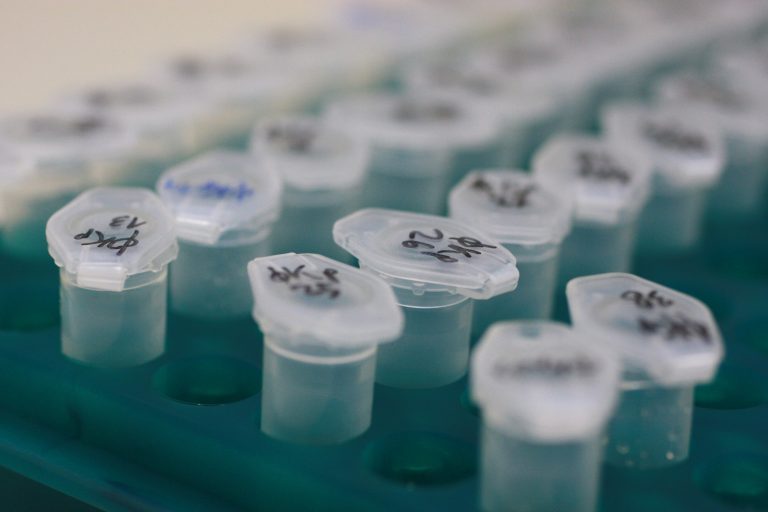 The Phage Library’s treasures. (Photo: TU Delft)
The Phage Library’s treasures. (Photo: TU Delft)The craftmanship from the early days of phage therapy has been replaced by genetic analyses, systematic screenings and databases. Microbiology has become an exact science.
Besides the fundamental screening work, the Fagenbank is in contact with medical staff from the UMC Utrecht who has applied for a clinical trial. They’re looking for an alternative therapy for pneumonia in patients with cystic fibrosis. Repeated antibiotic therapies in this patient group means that bacterial resistance is a common occurrence. The proposed clinical test will apply a mix of bateriophages that has shown positive against the pneumonia bacillus Pseudomonas aeruginosa.
Microbiologist Brouns expects the best results from sampling individuals. This implies taking a sample of the infection and determining the bacteria from its DNA. Once that’s known, the database can come up with matching bacteriophages. These will be fetched from the freezer, multiplied and applied to the patient.
However, such a targeted approach is at odds with the principle of the double-blind clinical test where all patients, except those receiving placebos, receive the same medication. Instead, this clinical test will see a broad selection of phages that have shown activity against Pseudomonas.
 (Photo: TU Delft)
(Photo: TU Delft)The Fagenbank is a particular initiative that relies on donations for funding. A starting subsidy of EUR 144,000 from the Delft University Fund got the collection started. It allowed Brouns to hire an analyst for 2.5 years. For further research, Brouns is on the outlook for additional funding.
Do you have a question or comment about this article?
j.w.wassink@tudelft.nl


Comments are closed.