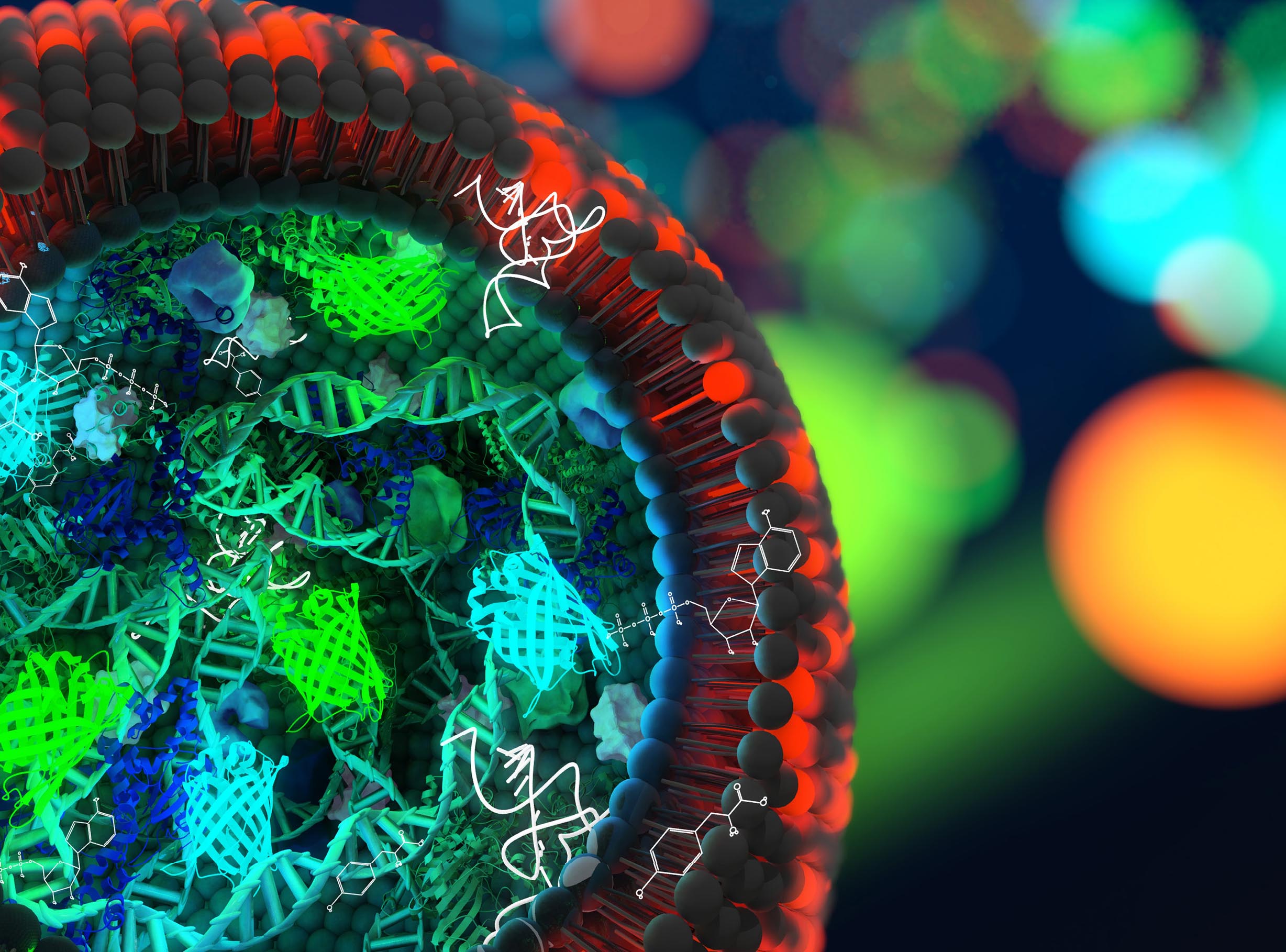
In the quest for creating artificial life, TU Delft researchers have taken an important step. They designed a strand of artificial DNA that is capable of copying itself.
The DNA strand only contains a couple of genes, just enough to kick-start the DNA replication process. Lacking the genes that regulate cell division and survival, talking about artificial life is an over statement. But the replication takes place in a small cell, a liposome.
“It is certainly a breakthrough,” says Dr Pauline van Nies, who until recently worked at the bionanoscience department in Delft, in the group of Prof. Christophe Danelon. Van Nies is the first author of a paper ‘Self-replication of DNA by its encoded proteins in liposome-based synthetic cells’, describing this feat. It was published this week in Nature Communications.
The Delft research builds in part on the work of a group of Japanese scientists who, about 15 years ago, assembled a cocktail of enzymes that can transcribe DNA, the process that ultimately leads to the production of new proteins. Feed these enzymes a strand of DNA with genes encoded for proteins that orchestrate DNA replication, and bob’s your uncle: the DNA will self-replicate. Or not?
DNA replication is extremely complicated
This sounds simpler than it is. DNA replication is an extremely complicated process. Van Nies thought of using the DNA replication machinery of a virus called Φ29. “Viruses are extremely efficient in encoding proteins in a small genome and in robustly replicating their genetic information.”
In human cells, DNA replication is managed by hundreds of proteins. Φ29 only needs four. The Delft researchers joined forces with microbiologist Margarita Salas and a couple of her colleagues at the Autonomous University of Madrid. Salas has been working with Φ29 for almost half a century. She discovered the DNA replication mechanism of the Φ29 virus and managed to isolate it.
Van Nies composed a unique DNA blueprint that took into account a number of different factors related to the flow of genetic information, such as a suitable binding site for the ribosome, an element that is essential for the production of proteins.
Cell division may be a tougher nut to crack
A goal that is now coming closer is combining the new module that regulates the flow of genetic information with other essential cellular functions such as growth and division. Last year, Danelon’s group created a way to synthesise the phospholipids that make up liposomes, such as the ones the researchers used in this project. The yield of phospholipids was still too small to sustain growth, but Danelon is confident his group can optimise this process.
Cell division may be a tougher nut to crack. Modern cells require a streamlined process in which copied DNA is neatly packed and then evenly distributed towards the poles of the cell. Concurrently, specialised proteins squeeze the mother cell into two daughter cells. Danelon thinks a simple ‘budding’ mechanism could also do the trick.
“I think we can create liposomes that grow until they start budding. If enough DNA is being produced, hopefully, enough of these primitive daughter cells will contain the new DNA to sustain a cell population”, says Danelon in a TU press release. This may well be how the very first cells self-reproduced before evolution equipped them with a more elegant and robust solution.
Do you have a question or comment about this article?
tomas.vandijk@tudelft.nl

Comments are closed.