Mopping up and storing CO2 from underground coal gasification projects is not feasible with current technology, says Dr. Ali Akbar Eftekhari in his PhD-thesis. It would cost more energy than it produces.
Turning coal into gas underground, as proposed by Sir William Siemens to the Chemical Society in London as early as 1868, is a tempting means of winning fuel without the need to go down a pit or to ruin the landscape. Ever since the first attempt in 1912 in the UK, interest in the technology neatly correlated with fuel prices and scarcity of alternatives.
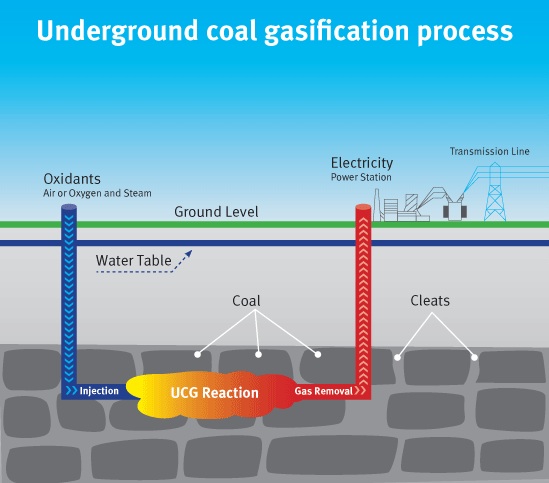
The technique basically consists of igniting coal seams underground and feeding the glow of 700 – 900 degrees Celsius with air (or oxygen) and/or steam from the surface. Out coming gases include carbon monoxide (CO), carbon dioxide (CO2), hydrogen (H2), methane (CH4) and various contaminants. The ratios depend on the feeding gases, pressure, and the quality of coal.
Europe still has considerable coal reserves in the UK, Poland and France for example, which could be won by gasification. The HUGE project (Hydrogen-orientated Underground coal Gasification for Europe) aims to make coal gasification competitive with other fuels in terms of CO2 emission.
This means some form of CO2 capture and storage has to accompany the gasification, for burning coal typically produces three times more CO2 than an equal amount of methane does. The combination of coal gasification with CO2-capture and storage has been dubbed ‘green coal’ – much to the anger of environmentalists who regard the term as an oxymoron (contradiction in terms).
In his PhD research the Iranian chemical engineer Dr. Ali Akbar Eftekhari has studied various ways of CO2 capture and storage in combination with coal gasification such as: binding CO2 underground with the synthetic mineral calcium-oxide (CaO); separation of CO2 from the product gas by CO2 capture and storage (CCS) technology and binding CO2 from the product gas with the natural mineral wollastonite.
But at the end of the day, none of these options are viable he says. The last option (wollastonite) might make sense from an energy point of view, but the process is too slow to be practical.
The other processes are simply too energy-hungry. With the current state of technology, CCS requires 30 – 55 percent of the combustion energy of the fuel. Combine that percentage with the energy needed for recovery and inevitable energy losses, and you’ll end up spending more energy in the retrieval than you’ll get out of it. That must be a HUGE disappointment.
“Right now, the state of the art carbon capture method is amine chemisorption, which is very energy intensive”, says Eftekhari. An alternative method of carbon capture with only a quarter of the energy need is membrane separation. Up until now, it only works in small-scale applications.
Eftekhari adds, “If CO2 capture technology becomes more affordable, that is: less energy intensive, we can expect to use coal with a low carbon footprint in the future, with an acceptable recovery factor. But for now in terms of greenhouse gas emissions, it’s better to use natural gas.”
–> Ali Akbar Eftekhari, Low Emission Conversion of Fossil Fuels with Simultaneous or Consecutive storage of Carbon Dioxide, PhD supervisors Prof. Hans Bruining and Dr. Karl Heinz Wolf (CEG), 26 September 2013


Comments are closed.